How AI helps in biotech therapies
- Daniela Vidal
- Feb 7, 2023
- 7 min read
Updated: Dec 13, 2023
I'm passionate about biology and biotechnology, so I was excited to write this blog.
I have three objectives with it: to learn about different technics in biotech therapy, find out some top biotech startups or companies researching this field, and find common points where Artificial Intelligence is helping these researchers.
For this purpose, I used the help of our beloved Chat GPT, asking the questions I had when I started to search for biotech companies in the Therapeutical areas. Making good questions about interesting topics turned out to be my superpower. So I hope you enjoy the reading.
------------------------------------
Artificial Intelligence (AI) has been making significant strides in various industries, and biotechnology is no exception.
In recent years, Artificial Intelligence in healthcare has been increasingly used in the therapy area of biotechnology, providing new insights and opportunities to improve patient outcomes.
From drug discovery and development to personalized medicine, AI is revolutionizing how we approach healthcare.
In this blog, we will explore three different biotechnology therapies; we will talk about three companies that are leading the research of Biotech therapies, the various ways in which AI is helping them, and how this technology is shaping the future of healthcare.
We will examine the role of AI in microbiome-deliver drugs, cancer therapy with viruses, and Kinase Therapy.
Whether you are a healthcare professional, a patient, or simply interested in the impact of technology on medicine, this blog will provide a comprehensive overview of how AI is changing the landscape of healthcare and improving patient outcomes.
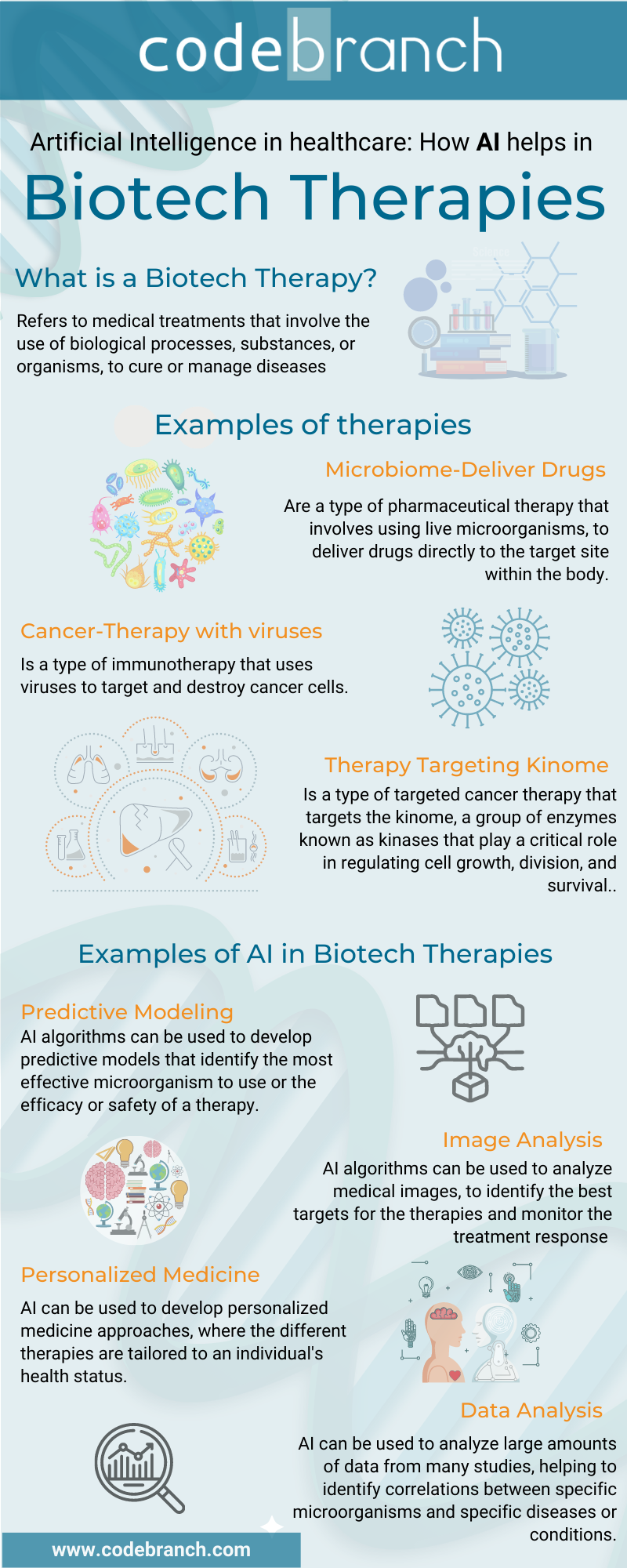
The use of AI in Microbiome Deliver-Drugs Therapy
What are microbiome-deliver drugs?
Microbiome-delivered drugs are a type of pharmaceutical therapy that involves using live microorganisms, such as bacteria or fungi, to deliver drugs directly to the target site within the body.
This approach is designed to harness the natural abilities of these microorganisms to colonize specific tissues, thereby increasing the efficacy and reducing the toxicity of the drugs being delivered.
This technology is still in its early stages of development but holds promise for treating various conditions, including cancer, inflammatory diseases, and infections.
What technological tools are used for developing microbiome deliver drugs?
Several technological tools are used in the development of microbiome-delivered drugs, including:
1. Synthetic Biology:
It involves the engineering of microorganisms to produce and deliver specific therapeutic compounds.
2. Metagenomics:
This is the study of the genomes of microorganisms present in a given sample, such as the human gut microbiome.
Metagenomics can be used to identify bacterial species that are well-suited for delivering drugs.
3. Microbial Fermentation:
This is the process of growing microorganisms in a controlled environment to produce large quantities of a desired therapeutic compound.
4. Microfluidics:
This technology involves the manipulation of small volumes of fluids, such as those found in microorganisms, for applications in drug development.
5. In vitro and in vivo models:
Cell-based and animal models are used to test the efficacy and safety of microbiome-delivered drugs and understand how these drugs interact with the host and target tissues.
These tools allow researchers to design and develop highly specific and compelling microbiome-delivered drugs that target specific diseases or conditions.
I found an interesting company working in this field, SFA Therapeutics. Its objective is to obtain drugs from metabolites.
This type of drug leads to the absence of genotoxicity, faster clinical development, and safer treatments.
Their pipeline includes treatments for psoriasis, liver cancer, ophthalmic diseases, cytokine release syndrome, relapse prevention in leukemias, and other diseases.
Their microbiome-derived drugs are derived from natural substances and enable a new platform to develop treatments that can potentially treat over 85 chronic inflammatory diseases currently afflicting patients, providing safer treatments than current therapies.
The use of AI in cancer therapy with viruses
How does it work Cancer-Therapy with viruses?
Cancer therapy with viruses is a type of immunotherapy that uses viruses to target and destroy cancer cells. This approach works by harnessing the natural ability of viruses to infect and replicate within cells. Here is a general overview of how it works:
1. Delivery of the virus:
The virus is delivered to the target cancer cells by injection directly into the tumor or systemically through the bloodstream.
2. Infection of cancer cells:
The virus infects the cancer cells, causing them to reproduce the virus and eventually leading to the cancer cell's death.
3. Stimulation of the immune system:
As the cancer cells die, they release viral particles and antigens, stimulating the patient's immune system to recognize and attack the remaining cancer cells.
4. Oncolytic replication:
Some viruses, known as oncolytic viruses, are designed to specifically target and destroy cancer cells without affecting healthy cells.
5. Combination with other therapies:
Cancer therapy with viruses can also be combined with other treatments, such as chemotherapy or radiation therapy, to increase the efficacy of these treatments and reduce the risk of side effects.
This approach to cancer therapy is still in its early stages of development. Still, it has shown promising results in preclinical and early-phase clinical trials, particularly for certain types of cancers, such as melanoma and lung cancer.
However, further research is needed to determine the safety and efficacy of this approach in more extensive, well-controlled clinical trials.
What technological tools are used for developing cancer therapy with viruses?
Several technological tools are used in the development of cancer therapy with viruses, including:
1. Genetic engineering:
This involves the manipulation of the virus's genome to enhance its ability to target and kill cancer cells while minimizing harm to normal cells.
2. Viral vector technology:
This technology uses virus-based delivery systems, known as viral vectors, to deliver therapeutic genes to cancer cells.
3. In vitro and in vivo models:
Both cell-based and animal models are used to test the efficacy and safety of virus-based cancer therapies and understand how these therapies interact with the host and target tissues.
4. High-throughput screening:
This involves the use of automated systems to test large numbers of viruses in parallel in order to identify those with the most significant potential for use in cancer therapy.
5. Immune profiling:
This involves the use of cutting-edge techniques, such as transcriptomics and proteomics, to understand the host immune response to virus-based cancer therapies, and to identify ways to enhance this response.
These tools allow researchers to design and develop highly specific and effective virus-based cancer therapies that target specific cancers or conditions while minimizing harm to normal cells.
I found a great and very promising company working in cancer therapy with viruses, which is called Humane Genomics.
These guys have a Virus Development Platform, and their approach to designing therapeutics ensures safety and efficacy in targeted cancer treatment.
The process has these steps.
1. Viruses are designed on a computer using their library of verified genetic sequences and virus features.
2. Code is synthesized into sequence-perfect fragments. This reliability cuts the steps and time required by the previous methods like CRISPR.
3. They assemble the DNA and make the viruses.
4. They test the viruses.
The use of AI in therapy targeting kinome
How does therapy targeting kinome work?
Kinome therapy is a type of targeted cancer therapy that targets the kinome, a group of enzymes known as kinases that, t play a critical role in regulating cell growth, division, and survival. These enzymes are often overactive in cancer cells, leading to uncontrolled cell growth and division.
Here is a general overview of how kinome therapy works:
1. Identification of target kinases:
Researchers use various techniques, such as genomic sequencing, to identify the overactive kinases in a particular type of cancer.
2. Development of kinase inhibitors:
Once the target kinases have been identified, drugs can be developed that specifically inhibit these enzymes, blocking their ability to regulate cell growth and division.
3. Delivery of the kinase inhibitors:
The kinase inhibitors are delivered to the target cancer cells, either orally or through injection, where they bind to the target kinases and inhibit their activity.
4. Inhibition of cancer cell growth:
By inhibiting the activity of the target kinases, the kinase inhibitors reduce the ability of the cancer cells to grow and divide, leading to cell death and regression of the tumor.
5. Combination with other therapies:
Kinome therapy can also be used with other treatments, such as chemotherapy or radiation therapy, to increase the efficacy of these treatments and reduce the risk of side effects.
Kinome therapy is a relatively new approach to cancer therapy. Still, it has shown promising results in preclinical and early-phase clinical trials, particularly for certain types of cancers, such as leukemia and lung cancer.
However, further research is needed to determine the safety and efficacy of this approach in more extensive, well-controlled clinical trials.
What technological tools are used for developing kinome therapy?
Several technological tools are used in the development of kinome therapy, including
1. High-throughput screening:
This involves the use of automated systems to screen large libraries of compounds to identify those that can effectively inhibit target kinases.
2. Structural biology:
This involves the use of techniques such as X-ray crystallography and nuclear magnetic resonance spectroscopy to determine the 3-dimensional structure of target kinases, which is essential for the design of drugs that can effectively inhibit these enzymes.
3. Computational biology:
This involves using computational methods, such as molecular docking and molecular dynamics simulations, to predict the interactions between kinase inhibitors and target kinases and to optimize the design of these inhibitors.
4. Cell-based assays:
This involves the use of cell-based systems, such as cancer cell lines and primary tumor cells, to test the efficacy of candidate kinase inhibitors and to determine their effects on cancer cell growth and survival.
5. Animal models:
This involves the use of animal models, such as mice, to test the efficacy and safety of candidate kinase inhibitors in a more complex and physiologically relevant environment.
These tools allow researchers to design, develop, and test kinase inhibitors that can effectively target the specific kinases that are overactive in a particular type of cancer, leading to the development of more targeted and effective kinome therapy.
In his field of Biotechnology for health care, I found Harmonic Discovery. They focus on creating a new class of drugs targeted against the human kinome, a family of 500 proteins associated with diseases such as cancer, auto-immunity, and neurodegeneration.
Their therapeutics is the result of integrating several layers of information, from single point mutations in protein sequence, through 3-dimensional conformational changes of protein structure, to systematic changes in protein gene expression… how Amazing!
If you are interested in creating a Start-up with a tech base, I recommend you to read: 5 steps to successful software development for start-ups.
How AI helps with Microbiome-deliver drugs, cancer therapy with viruses, and Kinome therapy?
In the next two charts, we summarized how Artificial Intelligence helps biotech health care; this tool is definitely a keystone for developing more effective and less harmful new drugs and treatments.
| Microbiome-Deliver Drugs | Cancer-Therapy with Viruses | Kinome Therapy |
Drug discovery/ Drug Design | AI algorithms can be used to design and optimize drugs for microbiome delivery, taking into account factors such as the stability and bioavailability of the drug, its ability to reach target tissues and its ability to avoid degradation by the host's immune system. | | AI algorithms can be used to analyze large amounts of data from high-throughput screening assays, structural biology studies, and computational biology simulations, helping to identify potential kinase inhibitors that can effectively target the specific kinases that are overactive in a particular type of cancer. |
Predictive modeling | AI algorithms can be used to develop predictive models that identify the most effective bacteria or fungi for delivering a specific drug, based on factors such as
| AI algorithms can be used to develop predictive models that identify the most effective viruses for a specific type of cancer, based on factors such as:
| AI algorithms can be used to develop predictive models that predict the efficacy and safety of potential kinase inhibitors based on factors such as
|
Data Analysis | AI can be used to analyze large amounts of data from metagenomic studies, animal models, and clinical trials, helping to identify correlations between specific microorganisms and specific diseases or conditions. | AI can be used to analyze large amounts of data from animal models, preclinical studies, and clinical trials, helping to identify correlations between specific viruses and specific cancers and optimize dosing and delivery strategies. | |
Personalized medicine | AI can be used to develop personalized medicine approaches, where the different therapies are tailored to an individual's health status based on data from their gut microbiome, tumor, and other sources. |
Image analysis | AI algorithms can be used to analyze medical images, such as CT and MRI scans, to identify the best targets for the therapies and monitor the treatment response. |
Conclusion
Artificial intelligence (AI) has been particularly impactful in the biotech industry. These technologies have the potential to revolutionize the way we approach healthcare by allowing for faster, more accurate, and more efficient diagnoses and treatments.
AI has the potential to significantly enhance the development of cancer therapy with viruses by providing insights into the complex relationships between viruses, cancer, and the host immune system and by helping to optimize virus-based cancer therapies for maximum efficacy and safety.
AI has the power to improve the development of kinome therapy significantly, by providing insights into the complex relationships between kinases, cancer, and the host and by helping to optimize kinome therapy for maximum efficacy and safety.
AI has the capacity to contribute to the development of microbiome-delivered drugs by providing insights into the complex relationships between the human microbiome, disease, and drug efficacy and by helping to optimize drug delivery and efficacy.
If you want to read more blogs about AI you can visit:
Comments